刘海峰1a, 1b,赵 键1b,王 华2,朱永昌2,李 婕1b,张行泉1a, 1b,郑 奎1a,霍冀川1a, 1b
(1.西南科技大学 a.环境友好能源材料国家重点实验室; b.材料科学与工程学院,四川 绵阳 621010;2.中国建筑材料科学研究总院有限公司 陶瓷科学研究院, 北京 100024)
DOI:10.13732/j.issn.1008-5548.2022.03.015
收稿日期: 2021-12-16, 修回日期:2022-03-31,在线出版时间:2022-04-25。
基金项目:国家自然科学基金项目,编号:51502249;四川省科技厅应用基础研究项目,编号:2020YJ0419;环境友好能源材料国家重点实验室开放基金,编号:18kfhg04。
第一作者简介:刘海峰(1983—),男,副教授,博士,研究方向为钙钛矿结构氧化物功能材料、核素晶格固化。E-mail:liuhaifeng@swust.edu.cn。
通信作者简介: 霍冀川(1962—),男,教授,硕士,博士生导师,研究方向为核素晶格固化。E-mail:huojichuan@swust.edu.cn。
摘要:为了寻求一种晶格更大、结构稳定的钙钛矿母相,实现对90Sr的有效晶格固化,以88Sr模拟90Sr,以锆基钙钛矿型氧化物BaZrO3为母相,采用溶胶-凝胶法制备Ba1-xSrxZrO3(0≤x≤1)固化体;通过系列表征,分析烧结温度、 Sr掺入量对固化体晶体结构、微观形貌及化学稳定性的影响。结果表明:采用溶胶-凝胶法成功制备了具有单一物相的钙钛矿型Ba1-xSrxZrO3,实现了对Sr的有效晶格固化;当x=1.0时,固化体对Sr的固溶量达到最大;半径较小的Sr2+进入A位取代Ba2+,导致Ba1-xSrxZrO3的晶胞体积和晶胞参数减小,但晶体结构稳定,并保持立方钙钛矿相;Ba1-xSrxZrO3固化体中Sr的7 d浸出率在10-4 g·m-2·d-1级别,低于目前主要陶瓷固化体中Sr的浸出率。
关键词:废物处理;锶;晶格固化;钙钛矿结构
Abstract:In order to develop a perovskite-type parent phase with larger lattice and stable structure to realize the lattice immobilization of 90Sr effectively, Ba1-xSrxZrO3(0≤x≤1) immobilization forms were synthesized by sol-gel method, with 88Sr used as simulated element and zirconium-based perovskite-type BaZrO3 used as a parent phase. The results show that the perovskite-type Ba1-xSrxZrO3 with a single phase can be successfully synthesized by so-gel method. Sr is immobilized effectively in the lattice position of the perovskite-type oxide. The maximum solid soluble amount of Ba1-xSrxZrO3 for Sr has been obtained, i.e., x=1.0. Sr2+ with smaller radius can enter the A position of perovskite-type Ba1-xSrxZrO3 to replace Ba2+, resulting in decrease of cell volume and lattice parameters, but the crystal structure is stable and maintain a cubic perovskite phase. The leaching rate(7 d) of Sr in Ba1-xSrxZrO3 is at the level of 10-4 g·m-2·d-1, which is lower than that in the current main ceramic solidified bodies.
Keywords:waste treatment; Sr; lattice immobilization; perovskite-type
参考文献(References):
[1]YANG Y S, WANG X F, LUO S Z, et al.Stability studies of[CsxBay][(Al3+, 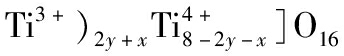
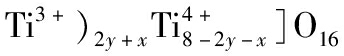 ceramics for radioactive caesium immobilization[J].Ceramics International, 2019, 45(6): 7865-7870.
ceramics for radioactive caesium immobilization[J].Ceramics International, 2019, 45(6): 7865-7870.
[2]李婷婷.普鲁士蓝类复合材料对放射性废水中Cs+和Sr2+的吸附性能研究[D].南京: 南京航天航空大学, 2017.
[3]GHOSH D B, KARKI B B, WANG J W.Utilization of artificial neural network to explore the compositional space of hollandite-structured materials for radionuclide Cs incorporation[J].Journal of Nuclear Materials, 2020, 530: 151957.
[4]LIU K, ZHANG K B, DENG T, et al.Heavy-ion irradiation effects of Gd2Zr2O7 nanocrystalline ceramics as nuclear waste immobilization matrix[J].Journal of Nuclear Materials, 2020, 538: 152236.
[5]MA J, FANG Z W, YANG X Y, et al.Investigating hollandite-perovskite composite ceramics as a potential waste form for immobilization of radioactive cesium and strontium[J].Journal of Materials Science, 2021, 56(16): 9644-9654.
[6]谢华, 冯志强, 王烈林.Er2Ti2O7烧绿石基玻璃陶瓷固化体的制备工艺研究[J].原子能科学技术, 2020, 54(1): 14-22.
[7]SHICHALIN O O, PAPYNOV E K, NEPOMNYUSHCHAYA V A, et al.Hydrothermal synthesis and spark plasma sintering of NaY zeolite as solid-state matrices for cesium-137 immobilization[J].Journal of the European Ceramic Society, 2022, 42(6): 3004-3014.
[8]YANG J X, SHU X Y, LUO F, et al.Solubility of Sr2+ in the Gd2Zr2O7 ceramics via appropriate occupation designs[J].Journal of Alloys and Compounds, 2019, 808: 151563.
[9]牟婉君, 李兴亮, 余钱红, 等.模拟核素固化体SrZrxTi1-xO3的化学稳定性[J].核技术, 2015, 38(9): 090301.
[10]KESKAR M, PATKARE G, SHAFEEQ M, et al.Structural and thermal study of Sr(Th1-xUx)(PO4)2 compounds[J].Journal of Solid State Chemistry, 2021, 300: 122228.
[11]张雪, 王进, 罗萍, 等.NZP型磷酸盐陶瓷固化模拟放射性核素Sr2+/Sm3+的研究[J].材料导报, 2022, 36(1):20090353.
[12]滕元成, 周时光, 肖正学, 等.Sr在碱硬锰矿固溶体中的化学固溶量研究[J].西安交通大学学报, 2005, 39(1): 100-103.
[13]American Society for Testing and Materials.Standard test methods for determining chemical durability of nuclear, hazardous, and mixed waste glasses and multiphase glass ceramics: the product consistency test(PCT): ASTM C1285-02[S].Philadelphia: ASTM, 2002.
[14]谢艳霞, 郑天辰, 石杰, 等.抗氧剂4010NA和RD对过氧化二异丙苯交联EPDM耐热复合材料性能的影响[J].高分子材料科学与工程, 2019, 35(10): 68-76.
[15]CEN J, LIANG F, CHEN D L, et al.Adsorption of water molecule on calcium fluoride and magnesium fluoride surfaces: a combined theoretical and experimental study[J].Journal of Physical Chemistry C, 2020, 124(14): 7853-7859.
[16]ZUBKOVA N V, NIKOLOVA R P, CHUKANOV N V, et al.Crystal chemistry and properties of elpidite and its Ag-exchanged forms[J].Minerals, 2019, 9(7): 420.
[17]SHANNON R D.Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides[J].Acta Crystallogr A, 1976, 32: 751-767.
[18]CAILLETEAU C, ANGELI F, DEVREUX F, et al.Insight into silicate-glass corrosion mechanisms[J].Nature Materials, 2008, 7: 978-983.

